The JnJ vaccine was tested in a large trial of almost 44 000 people from four continents, of whom 7000 participants came from South Africa. The follow-up time corresponded with our second wave, so the study was able to provide a good picture of how the JnJ vaccine works against the new 501Y.V2 variant, which is dominant in South Africa. This variant has been responsible for around 9 in 10 of all COVID-19 infections detected during the second wave and it is known to spread more rapidly than previous variants. The South African part of the trial showed that while the JnJ vaccine is not going to prevent mild symptoms, it provides 57% protection against moderate-severe disease, 85% protection against severe disease and 100% protection against death. By way of comparison the Oxford-AstraZeneca vaccine provided only 27% protection against mild to moderate COVID-19 caused by the new 501Y.V2 variant. The Oxford-AstraZeneca trial was not designed to investigate protection against severe COVID-19 and it remains unknown how effective it will be in doing this. As such, the Oxford-AstraZeneca vaccine may still play a valuable role in our Vaccination Programme in the future. The Oxford-AstraZeneca vaccine is additionally in the process of being updated to make it more effective against the new variants emerging all over the world. In the meantime, it is wise to start with a vaccine that we know protects against severe COVID-19 caused by the 501Y.V2 variant dominant in South Africa.
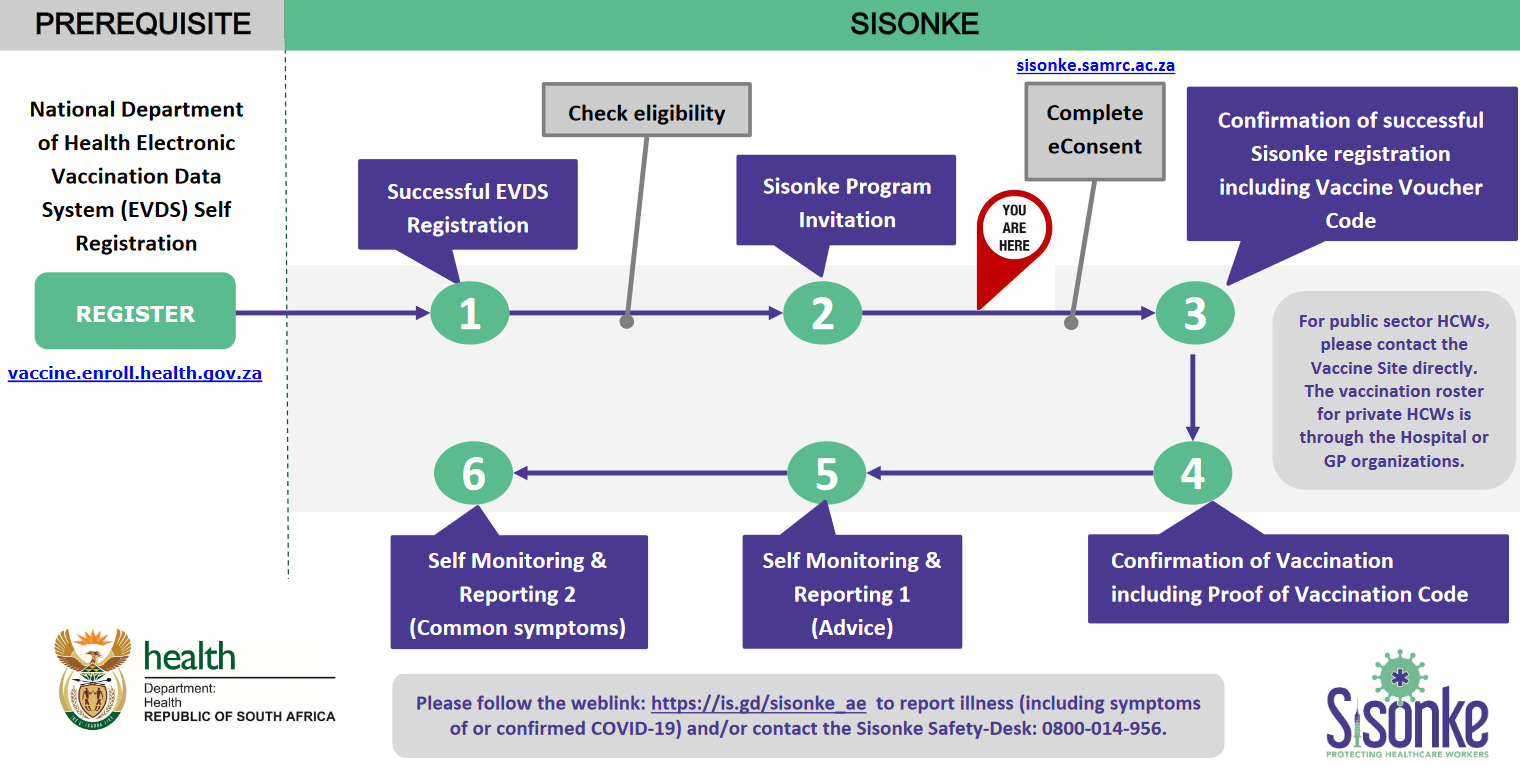
To be vaccinated in a hospital you need to be:
- • Age 18 and older AND
- • A health care worker in the private or public service AND
- • Willing and able to comply vaccination plan and other study procedures. AND
- • Capable of giving electronic or personal signed informed consent as described in Appendix 5, which includes compliance with the requirements in this protocol.